
Liming: Not All Lime Sources Are Equal – Part 3
Determining Lime Requirements and Liming Myths
Firstly, the quantity of lime required is not based on soil pH alone. Factors such as soil bulk density, the percentage of stone in the soil, liming depth, existing or potential crop are some examples that will help to determine and calculate lime requirements.
Secondly, the type of lime required. Although there are a variety of types of lime, in essence there are two main types of lime used in agriculture, namely, calcitic lime and dolomitic lime. These are both carbonates’ forms of lime.
Other lime sources include oxides and hydroxides (burnt or slaked lime). These other lime sources are often highly alkaline and are difficult to work with.
How does one determine the type of lime required? An accurate and in-depth soil analysis is the best way to determine the type of lime required. Not only will this provide some of the factors required to determine the amount of lime required but also indicates the quantities of calcium and magnesium in the soil. Calcitic lime and dolomitic lime are both calcium magnesium carbonates, with dolomitic lime having a higher percentage of magnesium than calcitic lime. By measuring the calcium : magnesium ratio of your soils, the correct form of lime can be applied.
Calcitic and dolomitic lime differ in the quantities of calcium and magnesium of which they are composed. The effectiveness and reaction speed of lime is also determined by other factors such as:
- Calcium carbonate equivalent (CCE)
- CCE: Measures a liming material’s ability to neutralise acidity by comparing its effectiveness to pure calcium carbonate (CaCO3 – 100%)
- Hardness (crystallinity and porosity)
- Calcium and Magnesium Content
- In South Africa, the requirements of ACT 36 OF 1947 states the following:
- Dolomitic Lime: > 4.3% Magnesium
- Calcitic Lime: < 4.3% Magnesium
- Porosity
- The higher the porosity, the greater the surface area of the limestone particle. Resulting in greater surface area for reactivity in soil.
- Fineness
- Ordinary agricultural lime: 50% finer than 0.25 mm
- Shell lime: 60% finer than 0.50 mm
- Microfine agricultural lime: 90% finer than 0.25 mm and 8 % finer than 0.106 mm
Combined with the CCE, the finer and more porous the lime, the quicker the reaction. This is due to an increase in surface area and contact with soil.
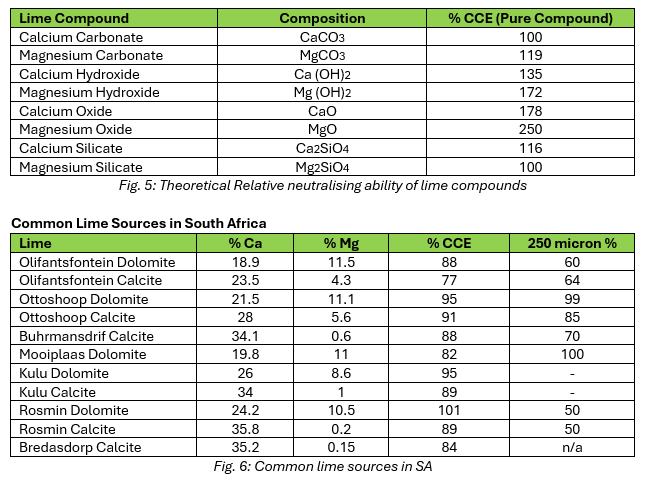
As evident in Figure 6, the lime sources in South Africa differ from each other significantly in composition, CCE and particle fineness. Deciding on the source of lime is dependent on the farm goal as well as cost, based on distance from site (transport cost), and the required reactivity time.
Although some sources of agricultural lime may seem cheaper due to proximity and transport costs, they may not be the best choice for the farming operation.

What About Granular Lime, Liquid Lime, and Gypsum?
Granular Lime and Liquid Lime
Over the past few years, granular lime has been marketed extensively as an effective alternative to conventional agricultural lime. The marketing of the product is often linked to claims such as
- “Granular lime only needs to be applied at a 10th of the conventional recommendation”,
- “As a granular microfine lime, it will react rapidly to change soil pH;” and
- “Granular lime is a good source of calcium for the crop.”
Study Findings
In a recent paper (Effect of Lime Source, Form and Placement on Soil pH Neutralisation and Permeation) published by Dawid Jacob Johannes du Toit from Stellenbosch University, it was found that granular lime was significantly less effective than regular lime. It was found that although granular lime is marketed as a “micro-fine” lime, the binding agent changes the properties, result in the lime to behave like a larger particle would. This results in a sharp change in pH around the granule, but there is no effective change between granules and through the soil profile. Combined with this, the granulated lime does not permeate well though the soil into the root zone due to only partial dissolution over time.
The study indicates that from a financial aspect, granular lime would not be a good financial investment as the price of the product is excessive and does not achieve the desired goal.
It must also be noted that the quantities of granular lime recommended are usually a 10th of the actual quantity required, therefore the rate of soil pH change will only be a 10th of its efficacy.
A common issue also encountered in South Africa, is fertiliser suppliers and farmers mixing granular lime with their fertiliser. This is an ill-advised practice due to chemical reactions that occur when the lime interacts with nitrogen, phosphorus and trace elements that may be present in the fertiliser.
Nitrogen fertilisers, especially ammonium-based fertilisers (LAN, CAN, MAP), begin to volatilise with these reactions, releasing ammonia gas into the atmosphere and essentially wasting fertiliser. Phosphorus and trace elements bound into complex molecules in the soil when exposed to rapid changes in pH close to the fertiliser. This makes these nutrients unavailable to the crop and therefore reduces the efficiency of the fertiliser.
Liquid lime is promoted in a similar fashion to granular lime, with marketing stating better efficiency than regular agricultural lime, lower quantities required (in some case as low as 1% of the recommended lime requirement), and a good source of crop nutrition.
The effects of liquid lime are short lived, requiring more frequent applications at a greater cost and decreased efficiency due to factors linked to CCE, fineness, particle distribution, and soil incorporation.
Gypsum
Gypsum (calcium sulphate) is often confused as a lime source. Gypsum in fact has no effect on soil pH in the rooting zone.
As stated earlier, there is a misconception that calcium is responsible for pH change, where in fact it is the carbonate component that reacts to increase soil pH. With gypsum being calcium sulphate, there is no carbonate component to modify pH.

Some agronomists may recommend gypsum and agricultural lime simultaneously; this practice again follows the Albrecht Method of calcium and magnesium base saturation percentages. This comes at the risk of under or over liming and the addition of excessive amounts of gypsum.
It is important to note that gypsum is a neutral salt which can increase calcium and sulphur levels in soils for plant nutrient uptake. This does come at a risk though. At high levels of gypsum application (excess of 500kg / hectare), the calcium ions can replace other cations on the soil exchange sites resulting in leaching of other nutrients. Typically, this results in leaching of sodium, but if sodium levels are low in soil, it can result in leaching of magnesium and potassium.
It is important to have a full analysis of a soil sample to understand the implications of applying gypsum.
