
Liming: Not All Lime Sources Are Equal – Part 1
An Introduction to Soil pH and Liming
Lime reacts with soils to increase the soil pH to a more desirable point. At soil pH levels closer to neutral, most essential nutrients become more available to the plant, there is less risk of fertiliser losses and beneficial soil biology tend to favour these conditions.
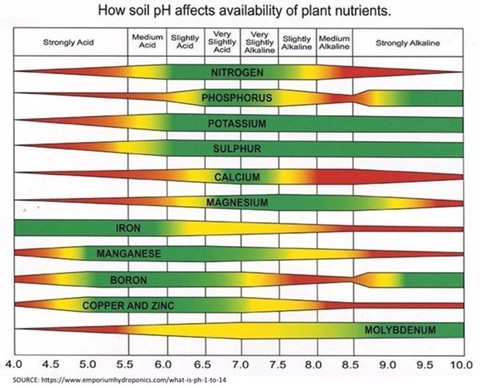
Fig. 1: Effect of soil pH on plant nutrients
As indicated in the image above (Fig.1), soil pH dramatically affects plant nutrient availability at both low soil pH (extreme acidity) and at high soil pH (extreme alkalinity). Under acidic conditions, there are a variety of factors that can limit plant nutrient uptake.
Acidic Soil pH Effect on Plant Nutrient Availability
- Nitrogen: Nitrogen is inhibited through decreased activity of beneficial nitrifying bacteria activity. This reduction of nitrification results in poor conversion of ammonia to the plant available nitrate form.
- Phosphorus: Phosphorus binds to iron and aluminum under acidic soil conditions. Making it less available to the crop.
- Potassium, Calcium and Magnesium: Potassium, calcium and magnesium availability decreases at low soil pH due to competition for exchange sites on soil particles with hydrogen ions and toxic soluble aluminum ions. This increases leaching risk of potassium by displacing it into the soil solution.
- Trace Elements: Some trace elements increase in availability at lower soil pH, while others decrease, especially molybdenum. At extreme low soil pH some elements like manganese and copper can become toxic to crops, which inhibits root development and nutrient uptake.
- Reduced CEC: Low soil pH decreases the CEC of soils, which reduces the soils’ ability to hold and supply nutrients to the crop.
- Aluminum Toxicity: Soluble aluminum becomes more abundant at low soil pH. At toxic levels, aluminum can inhibit root growth and reduce the plants’ ability to absorb nutrients.
- Soil Biology: Low soil pH can inhibit beneficial soil biology, which disrupts nutrient cycling, enzyme activity and relationships between roots and beneficial bacteria (rhizobia) and fungi (mycorrhizae).
Acidic Soil pH Effect on Fertiliser
As seen in figure 2, as soil pH decrease, there are dramatic losses in fertiliser availability.
These losses are through similar processes mentioned under the effects of soil pH on plant nutrient availability. Soil biology is inhibited, binding of nutrients into insoluble forms and competition at exchange sites.
With current fertiliser prices as they are, farmers cannot afford to have soils at the lower end of the soil pH spectrum.
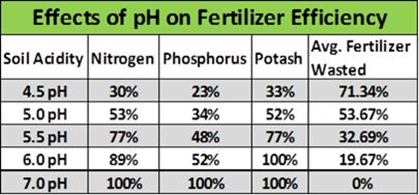
Fig. 2: Fertiliser wastage at low soil pH
