
Liming: Not All Lime Sources Are Equal – Part 2
Understanding Soil pH
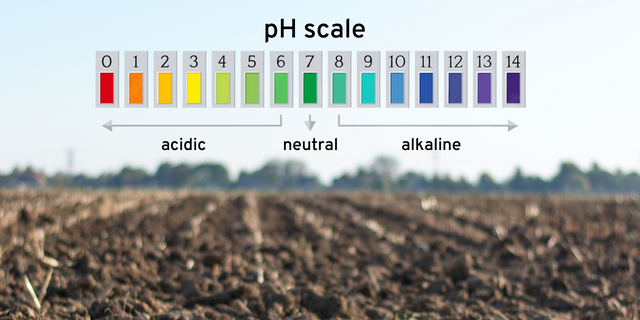
Soil pH is a chemical measurement of how acidic or alkaline soil is. This is based on the negative logarithm of hydrogen ion (H+) concentration. As a logarithmic scale, a change of one whole pH unit represents a tenfold difference in acidity or alkalinity. For example, a soil pH of 5, is 10 times more acidic than a soil with a pH of 6, and 100 times more acidic than a soil pH of 7.
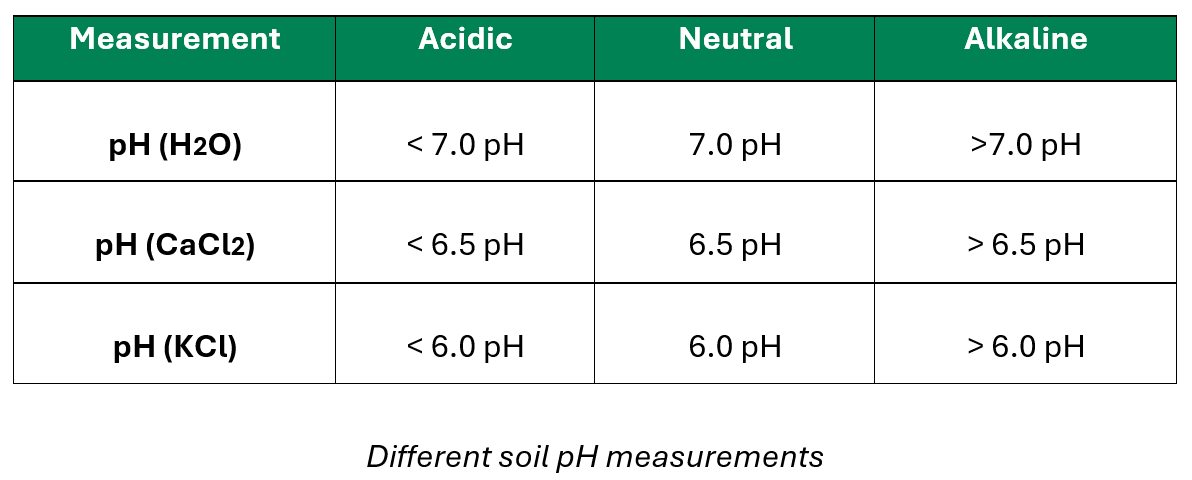
Note that soil pH can be measured in three different ways – water pH (pHH2O), calcium chloride pH (pHCaCl2), and potassium chloride pH (pHKCl). Each a slightly different neutral point, which is important to know when evaluating soil pH. Most laboratories will supply soil pH results as pH (KCl), but some laboratories will supply the information in one of the other formats.
It is also important to understand that although these differences might seem minor, they have a significant impact on the efficacy of the lime applied.
pH (H20) and pH (KCl) are the predominant pH measurements encountered. The two differ dramatically in interpretation.
- pH (H20):
- Measures: The pH of the soil solution, which is the active acidity or alkalinity.
- pH (KCl):
- Measures: The pH of the soil solution (immediate acidity or alkalinity) and the reserve acidity (tied to the CEC – residual and exchangeable acidity)
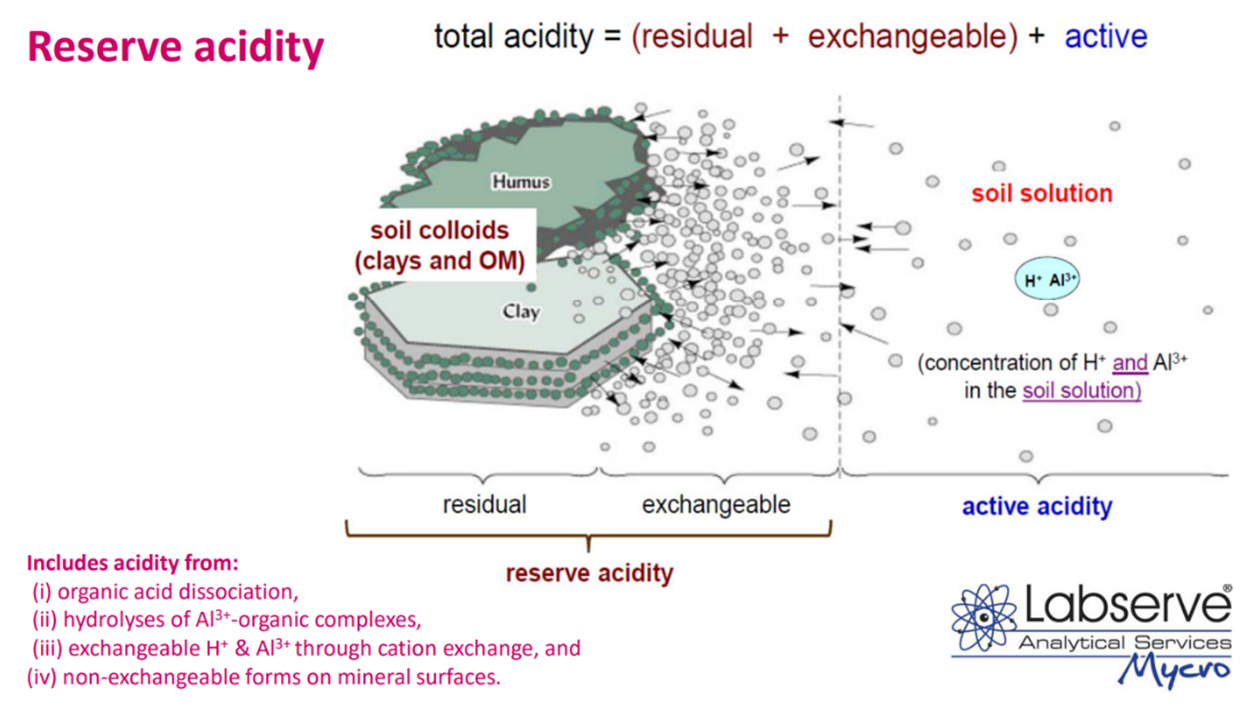
This explains why pH (KCl) measures more acidity and lowers the neutral point. This is important to understand, as without measuring the reserve acidity, quantities of lime applied may be incorrect as soil has the ability to “buffer” pH changes.
The soil pH buffer capacity is the soils’ ability to resist changes in soil pH and maintain a relatively stable pH even when alkaline substances like lime are applied. If lime is under applied, there may be an initial change in the active acidity, but this change is resisted by replacement the of ions from the residual and exchangeable acidity (reserve acidity) in the soil solution and on the soil colloid, thereby negating the effect of the lime – The Buffering Capacity.
How Does Agricultural Lime Increase Soil pH?
There is a common misconception that calcium is the neutralising agent involved in increasing soil pH.
It is in fact the carbonate interaction in the soil that results in the reactions in the soil. Carbonates neutralise soil acids by reacting with the abundant hydrogen ions (H+) in an acidic soil, converting them into water (H₂O) and carbon dioxide gas (CO₂). This reaction removes the acid from the soil solution, increasing the soil’s pH and making it less acidic.
Reactions in Soil
- CaCO3 + MgCO3 + 2H2O + 2CO2 → Ca(HCO3)2 + Mg(HCO3)2
Carbonates (agricultural lime) are applied to soil. The carbonates react with water and carbon dioxide to form soluble bicarbonates.
- Ca(HCO3)2 + Mg(HCO3)2 → Ca⁺⁺ + Mg⁺⁺ + 4HCO3
These bicarbonates further react with hydrogen ions (H+) in the soil solution. These hydrogen ions are responsible for acidity in the soil.
- H⁺ + HCO3⁻ → H2CO3 ⇄ H2O + CO2
This reaction forms carbonic acid, which is unstable and quickly dissociates into water and carbon dioxide. This process consumes hydrogen ions from the soil solution, reducing the overall acidity.
Calculating Lime Quantity
There are various formulas used to calculate lime requirements in South Africa.
- Eksteen Method: Developed in the Western Cape, this method determines lime requirements based on the ratio of exchangeable calcium and magnesium to exchangeable acidity. Variations of the method exist.
- Cedara Method: This method, developed in KwaZulu-Natal, is based on acid saturation – the measure of exchangeable acidity in soil.
- pH % Clay Tables: A linear scale used to determine lime requirements.
- Incubation Method: Considered the most accurate method but requires a lot of time and lime being applied needs to be used in the test.
Study Findings
In a study (An Evaluation of Lime Requirement Methods for Selected South African Soils) performed by Vincent van der Berg from Stellenbosch University, the Standard Eksteen, Eksteen-KCl, Modified Eksteen and Cedara Methods were compared. Through this study he was able to determine that other factors, such as carbon, titratable acidity, buffer CEC and texture, have a significant impact on liming requirements.
He also concluded that the Modified-Eksteen Method was the best method across various soils across South Africa. The Standard Eksteen method had limitations on soils with high carbon levels, whilst the Eksteen-KCL and Cedara methods had consistent underestimations of lime requirements.
Unfortunately, very few agronomists use the Modified-Eksteen Method, resulting in under application of liming or in the case of the pH % Clay Table, an over application.
Another factor negated from many liming methods is stone fraction. Stone fraction can vary across soils, with some Western Cape soils having stone fractions in excess of 85%. When applying agricultural lime, a higher stone fraction requires a lower lime requirement. If not taken into consideration, this lack of critical data can often lead to over liming to the detriment of the soil and thus the production of the crop.
Some agronomists also incorporate the Albrecht Method into their recommendations, which is based on the base saturation calcium and magnesium percentages. This method is not used for calculating lime requirements, but agronomists may try to use agricultural lime to achieve the percentages that they desire. This method is outdated (based on data from prior to the early 1900’s) and can result in over application of agricultural lime in the goal to reach the “ideal percentages”.
Timing of Lime Application
Timing of lime application is just as important as the timing of fertiliser application for a crop.
When lime is applied to soil there is initially a rapid change in pH around the lime particles before it begins to diffuse through the soil. This rapid pH change can have a negative impact on fertilisers if applied closely to fertiliser applications.
If nitrogen fertiliser is applied soon after liming, there is a large risk of volatilisation of nitrogen (nitrogen loss into the atmosphere).
Phosphorus is also negatively affected by fertilising soon after liming. Phosphorus fertiliser has a short bioavailability time when applied, but by reacting with lime this time frame is reduced dramatically, resulting in a rapid “lock-up” of phosphorus. Most trace elements are also prone to be temporarily “locked up” by liming.
Recommendation
Due to this we recommend that liming be timed to be in the “quiet” time of a crop cycle. A minimum of 6 weeks prior to fertilisation or 2 weeks post fertilisation can be considered safe liming time for row crops. In orchard crops, winter liming is advisable.
